Introduction: While survival for patients with newly diagnosed CLL has improved over the past decade, investigations suggest that benefits have largely accrued among younger adults, with much more modest benefits observed in older adults. The objective of this study was to describe the prognosis of adults diagnosed with CLL at age 66 and older overall and by demographics, comorbidities, and markers of frailty.
Methods: We used Surveillance, Epidemiology, and End Results (SEER)-Medicare data to identify older adults (≥66 years) diagnosed with primary CLL (ICD-O-3: 9823) between 2004 and 2015 (the overall cohort). A subset of this cohort who initiated CLL-directed therapy following diagnosis was identified (the treated cohort). In both cohorts, patients were required to have continuous enrollment in Medicare Parts A and B and no managed care during the year following diagnosis. For the treated cohort, patients were also required to have continuous enrollment in Medicare Part D if they initiated treatment on or after January 1, 2007 (when Part D claims became available). To account for variable time between diagnosis and treatment initiation, a landmark approach was employed; only patients who survived at least one year following diagnosis (i.e., the landmark) were included in the analysis. For the treated cohort, only patients who initiated treatment within one year of diagnosis were included. Patients were categorized as having low, medium, and high comorbidity using the NCI Comorbidity Index and a high probability of being frail using Medicare claims algorithms in the year between diagnosis and the landmark. Patients were followed for CLL-specific and other-cause mortality from the landmark until the end of study (December 31, 2016). The cumulative incidence of CLL-specific and other-cause mortality was estimated overall and by patient demographics, comorbidity and frailty using the Aalen-Johansen estimator. Associations between patient demographics, comorbidity, and frailty and CLL-specific and other-cause mortality were assessed using Fine and Gray subdistribution hazards regression.
Results: The overall cohort and treated cohort included 12,687 and 1,543 CLL patients, respectively. The mean age at diagnosis (SD) in the overall cohort was 77 (7.3) years and 45% were female. In the overall cohort, 46%, 26% and 28% of patients were identified as having low, medium, and high comorbidity, respectively, with the most common comorbidities being uncomplicated diabetes (25%), chronic pulmonary disease (17%), and congestive heart failure (14%). Twenty-six percent of patients were identified as having a high probability of being frail (≥10%). Distributions of patient characteristics were similar in the treated cohort, with slightly higher proportions of medium (30%) and high comorbidity (34%) and high predicted probability of frailty (30%).
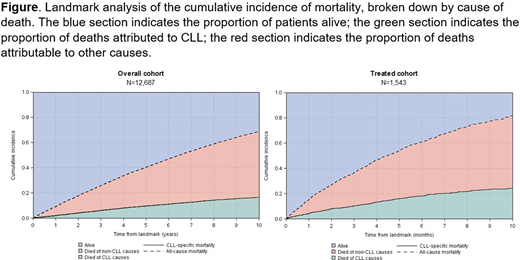
The 10-year cumulative incidence of mortality from the landmark was 69% (95% confidence interval (CI), 67-70%) in the overall cohort and 82% (95% CI, 79-85%) in the treated cohort, with most deaths attributed to non-CLL causes (Figure). In the overall cohort, older age at diagnosis, earlier year of cancer diagnosis, higher predicted frailty, and higher comorbidity were associated with worse prognosis. Notably, the associations between frailty and comorbidity and other-cause mortality (high vs. low frailty: hazard ratio (HR)=2.20, 95% CI: 2.00-2.43; high vs. low comorbidity: HR=2.00, 95% CI: 1.85-2.17) were stronger than their associations with CLL-specific mortality (high vs. low frailty: HR=1.43, 95% CI: 1.20, 1.70; high vs. low comorbidity: HR=1.07, 95% CI: 0.93-1.24). Results of the subdistribution hazards regression analyses were similar in the treated cohort. In analyses stratified by comorbidity level, frailty remained associated with mortality.
Conclusions: Most older adults diagnosed with CLL die from non-CLL causes. CLL-directed treatment decision-making in older adults should explicitly consider age-related health conditions, such as comorbidity and frailty, as they are strongly and independently associated with prognosis.
Duchesneau:AbbVie: Research Funding. McNeill:AbbVie: Current Employment, Current equity holder in publicly-traded company. Tyczynski:AbbVie: Current Employment, Current equity holder in publicly-traded company. Schary:AbbVie: Current Employment, Current equity holder in publicly-traded company. Lund:AbbVie: Research Funding; GlaxoSmithKline: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal